Table of Contents
palynology: the pollen database
Palynology is the study of pollen grains and other spores. Determination of the pollen, brought by the bees to the hive, can help us to map the foraging areas of the bees. It can give us useful information on the environment, for a wide range of purposes.
The pollen database is a project in collaboration with Masatoshi Funabashi from the Sony Research Lab in Tokyo.
June 2013 Masa and me decided to work on a joined research project that investigates the link between insects, pollen and ecosystems.
We will set up a database and compare pollen -straight from the plant- with pollen brought back by honeybees to the hive. With microscopy photography and pattern recognition software we hope to collect, compare and exchange information about the ecosystems foraged by the honeybees (and other insects).
pollen data sheet format
visualisation tools, scientific maps
We will work with the software ELFE to discover emergent patterns in a multitude of pollen pictures.
In philosophy, systems theory, science, and art, emergence is the way complex systems and patterns arise out of a multiplicity of relatively simple interactions. Emergence is central to the theories of integrative levels and of complex systems.
emergence
IT-Mediated Development of Sustainable Agriculture Systems
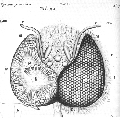
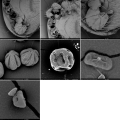
pollen analyse #1
pollen analyse #2
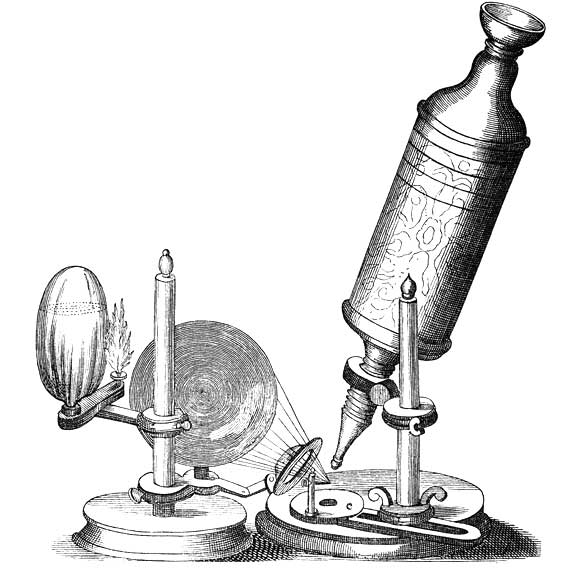


Compound microscope of Robert Hooke. The microscope was largely made of cardboard and was covered in dark red leather ornamented with gold stampings. The eye-lens sat in a wooden holder with the field-lens mounted inside the tube. A small oil lamp was used to shine light onto the specimen. With this hand-built object of cardboard, leather and wood, Hooke changed the way we see the world around us.
microscope history
Colourchart used by Ferdinan Bauer for his field studies - 1800.
usefull information
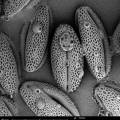
31/07/2013: Foeniculum vulgare (wild fennel) - Apiaceae


sources:
http://www.geo.arizona.edu/palynology/nsw/shimeld/foeniculum_vulgare.jpg
http://remf.dartmouth.edu/pollen2/pollen_images_3/index.html


sources:
http://www.botanicalgarden.ubc.ca/potd/2007/07/foeniculum_vulgare_and_rhagonycha_fulva.php
petal Foeniculum vulgare: photo annemie maes
31/07/2013: Fagopyrum esculentum (buckwheat) - Polygonaceae
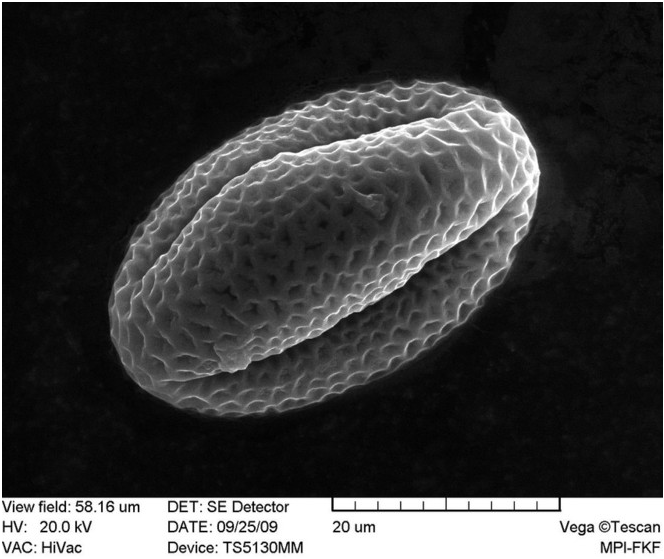
sources:
http://pollen.jimdo.com/galerie/pollen/pollen-b/#Buchweizen
photos microscopy series (Fagopyrum esculentum): Masatoshi Funabashi
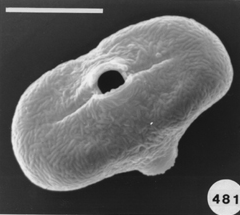
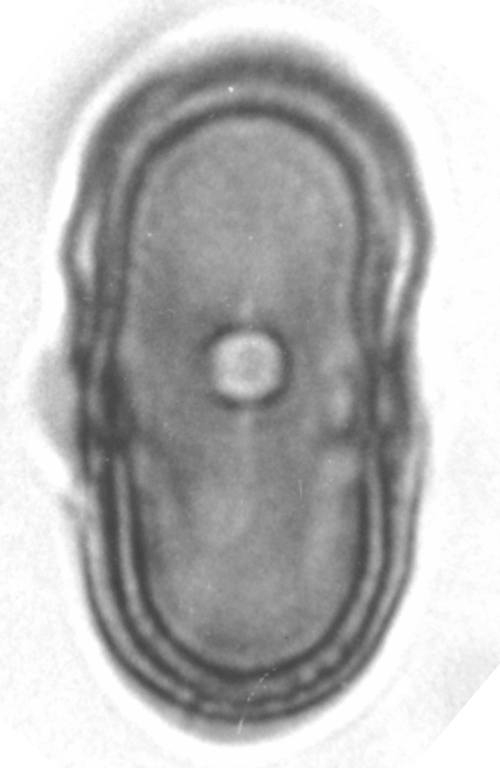
31/07/2013: Daucus carota subsp. sativus (carrot) - Apiaceae
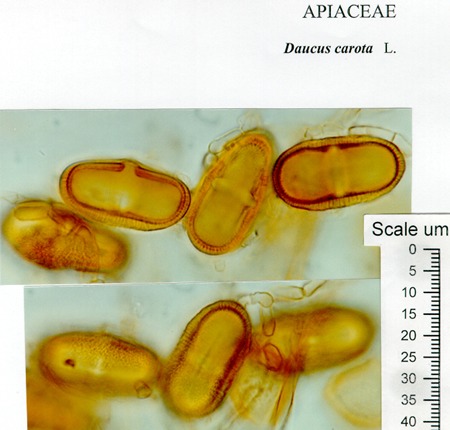
sources:
http://www.geo.arizona.edu/palynology/nsw/shimeld/daucus_carota.jpg
http://pollen.usda.gov/AtlasSEMPlates/Plates%2081_90/Plate%2082.htm
http://pollen.usda.gov/Light_Micrographs/Apiaceae/Apiaceae.html
photos microscopy series (Daucus carota): Masatoshi Funabashi
19/08/2013 : Masatoshi Funabashi
Je viens de recevoir un feedback d'un ingénieur de SONY qui s'occupe de ELFE.
Il dis que avec la précision des photos des pollens que je t'ai envoyé
l'autre jour, on peut compter une haute précision de classification:
la variation des traits(forme, couleur, taille etc) parait suffisant
pour l'apprentissage.
21/08/2013: pollen, flowers and bees
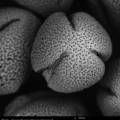
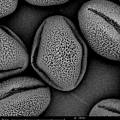
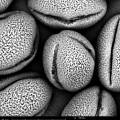
On 21/22/23-8 I can work at the Chemical Engineering Lab of the VUB on the SEM (Scanning Electron Microscope). The SEM offers the possibility to make perfect 3D images at +20.000 enlargment scale. Ideal for photographing pollen and bee-parts as proboscis, receptors, e.g.
The lab is specialized in surface metals research. I work with Gizem Süngü, a future PhD student.
The last weeks I colletected yet on several days pollen at the entrance of the beehives. I also have a pollen collecten from spring this year. We start with the spring pollen: olive green and light yellow. The SEM microscope operates in steps. We have to zoom in more and more, one (big) step at a time. The electrons are flying around and they are scanning the pollen. The bundles of electrons are manipulated by electrical fields; they create a similar effect as lenses on light. Finally the image is processed on a screen.
There are different setting possible. TIFF and Jpeg, different resolutions. Max. is 2048. We can play with contrast and brightness. When we go in higher magnifications, there is damage caused by the electrons. They are attacking our subject!
The pollen give a rather grey contrast. Their skin is hard and soft at the time. We are working with living material (not with dead metals!) and sometimes the subject disappears while we try to photograph it. Shot away by the electrons!
Alex Lutz tells us that we need to put a slight gold coating to prevent damage. He takes us to the 5th floor where the platinum coating machine is situated. A short introduction follows. The result afterwards is indeed much better. The resolution of the subject increases. The soft pollen and bee-parts become also better resistant to the shooting electrons.
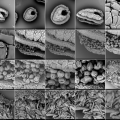
We try out different pollen combinations. Spring - I think the majority comes from the salix family - range of light yellows - 1). We pinpoint also a bright yellow pollen from spring - my guess is the Taraxacum officinale. And then a series of possibilities for the olive green pollen: Viburnum tinus 2), Rubus 3), Prunus avium 4), the yellowish green of the Malus domesticus 5). The olive of the Prunus spinoza 6), the fresh green of the Sambucus nigra 7), the darker green of the Vicia faba 8) and the olive green pollen of the Quercus robur 9). We need to do a lot of determination, but all these trees, flowers, herbs and vegetables are in and around the rooftop garden … A fascinating world is opening up. We try to organize as good as we can, being newbees in this SEM-world.
Than comes the bee-parts. Great! A bee eye 10) with 6900 hexagon pixel lenses, with hairs to protect them. The hexagon basis of the lenses are identical to the form of a honey comb. The antennae -the bees' instrument to scan the environment on all sensitory levels- are full of receptors - thousands of them! With these receptors, the bees sense an smell. 11).
Without the platinum coating these sensor-hairs were catapulted away and flattened by the electrons, but after coating we can photograph them in detail. Also the wing of the bee seems fascinating, with the hooks, the veins, … Not to speak about the proboscis 12) : this is truly a completely unknown world opening up. Scary and fascinating - makes me think of the jaws of a whale … at the end of the proboscis -the 3fold bee tongue- we can see the honey spoons, the bee-instrument for collecting the nectar out of the flowers. The minimum magnification of the SEM is x270. This makes that we are always much zoomed in, making an overview is difficult.
22/8/2013: working with prepared pollen samples
At the end of day 1 I came to following conclusion: it will be very difficult to determinate the pollen collected by the bees. I need another method for catalogueing the pollen. I will do a double check.
This morning, 22/8, I go out in the garden to collect pollen myself, straight from the flower. This is the real ALOTOF. A Laboratory On The Open Fields. I collect Calendula officinalis, Fragaria, Borago officinalis, Fagopyrum esculentum, Mentha spicata, Helianthum annuus, Cucurbita pepo, Solidago canadiensis, … (many more - fill out).
At the VUB Chemical lab I make samples of all this - dip the sticky tape in their pollen and put them on the little black dot to be scanned by the electrons. Making a good sample is the basis of a good image. Tomorrow is another day.
we make some pictures of samples taken on august 20 2013. In the results, we see that there is indeed a big difference between fresh pollen and dried pollen (the ones from april 2013). The fresh pollen move: they implode, as they are deshydratating. This happens via the pollen apertures, the slits we see in polair and equatorial view. The pollen can swell again as well if necessary, for example when they arrive via pollination on the pistil of the (female) flower and they grow via the pollenbuis and the style till they reach the ovary where fertilisation takes place. A seed is born.
Dehydrating fresh pollen - water is leaking out of the pores and the apertures, after a few days.
31/08/2013: final poster
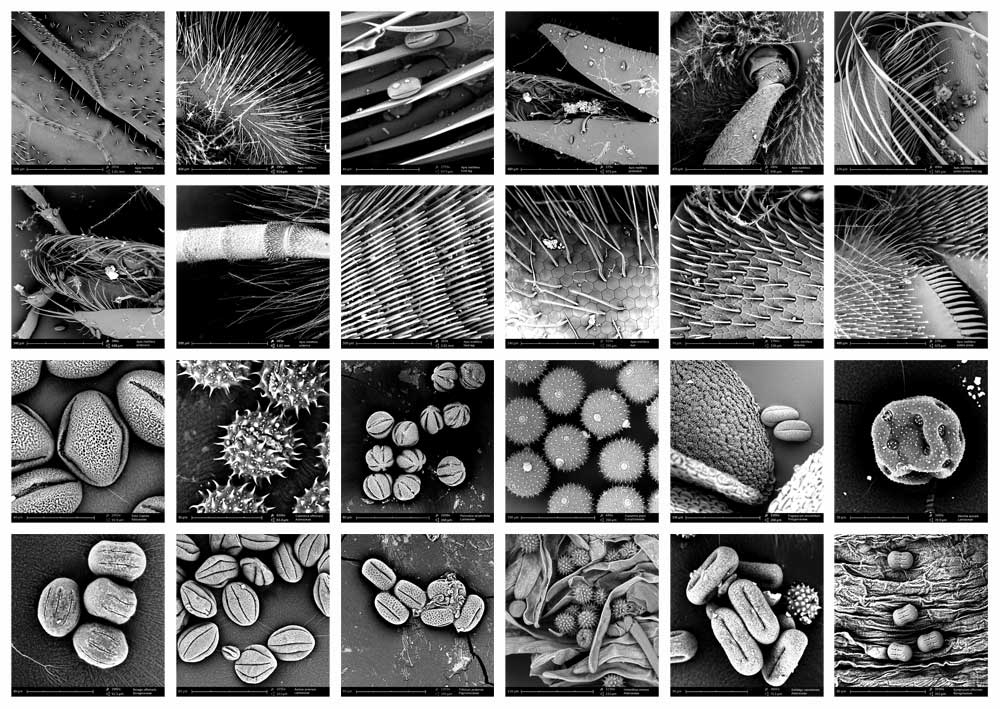
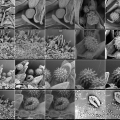
collection of Apis mellifera and pollen images made with SEM microscope, august 2013 (Annemie Maes and Gizem Süngü at chemical engineering lab, VUB Brussels.
more pollen info
01/3/2014: link via Yves Mostien
http://www.vcbio.science.ru.nl/virtuallessons/pollenmorphology/
04/03/2014: via Xavier Bellès from IBE institute (PRBB Barcelona)
A molecular approach to species identification of Chenopodiaceae pollen grains in surface soil

Abstract
Pollen identification and classification are important not only for palynologists, but also for systematists and ecologists. Because palynological methods for the identification of pollen in surface soil until now could resolve at best to the generic level, we have developed a molecular approach to species-level identification of Chenopodiaceae pollen in surface soils. Surface soil samples were collected in the central area of Junggar Desert Basin, Xinjiang, China. Fresh leaves of 19 Chenopodiaceae species were sampled for DNA sequencing, establishing a database of internal transcribed spacer (ITS) regions of nuclear ribosomal DNA for Chenopodiaceae. Individual chenopod pollen grains in a soil sample were separated from the soil and the ITS1 region of each pollen grain was amplified using nested PCR and sequenced. By comparing the amplified ITS1 sequences to those in the Chenopodiaceous database, we identified the pollen in the soil samples to the level of species. The new method provides a technical reference for species identification of soil surface pollen for other families. This work is necessary for further efforts to interpret the relationship of surface soil pollen to vegetation characteristics.
Pollen foraging behaviour of solitary Hawaiian bees revealed through molecular pollen analysis
Obtaining quantitative information concerning pollinator behaviour has become a primary objective of pollination studies, but methodological limitations hinder progress towards this goal. Here, we use molecular genetic methods in an ecological context to demonstrate that endemic Hawaiian Hylaeus bees (Hymenoptera: Colletidae) selectively collect pollen from native plant species in Haleakala and Hawaii Volcanoes National Parks. We identified pollen DNA from the crops (internal storage organs) of 21 Hylaeus specimens stored in ethanol for up to 3 years. Genetic analyses reveal high fidelity in pollen foraging despite the availability of pollen from multiple plant species present at each study site.
screenshots from the SEM-micrographs (library 2013-1)
The bee can detect a spatial odor pattern (3D odor shape of the flower). It can distinguish different concentrations applied simultaneously to the 2 antennae. After landing on the flower, the antennae smell various odors on the way to the nectar. Insects and Flowers - the Biology of a Partnership