This is an old revision of the document!
Table of Contents
dyeing with bacteria
Conference non-human agents, art laboratory Berlin (2017)
http://www.artlaboratory-berlin.org/html/de-event-40.htm
Ted Talk - How Bacteria Talk:
https://www.ted.com/talks/bonnie_bassler_on_how_bacteria_communicate
Bacterial dyes in Fashion:
https://www.asm.org/index.php/general-science-blog/item/6929-bacterial-dyes-in-fashion
Janthinobacterium lividum (Indigo):
https://en.wikipedia.org/wiki/Janthinobacterium_lividum
Streptomyces coelicolor (blue):
https://microbewiki.kenyon.edu/index.php/Streptomyces_coelicolor
Bacteria and Art:
https://microbewiki.kenyon.edu/index.php/Bacteria_and_Art:_Creation,_Deterioration,_and_Preservation
Living Color: Bacterial Pigments
http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1000510
growing Janthinobacterium lividum
LB Medium (250ml gedemineraliseerd water; 8,75gr LB powder; 5gr glycerine; mengen en autoclaveren).
Optimum growing conditions for Janthinobacterium: 25°C.
Glycerine is enhancing the growth of Janthinobacterium; Glucose is inhibiting the growth.
Bacteria on sterilized cellulose skin (grote petri-schalen): 1ste poging (met gesterelizeerde skins) is mislukt - kan ook liggen aan slecht groeimedium. 2de poging (LB met glycerine en nieuwe inoculatie van bacteria) is in wording.
Nieuwe test met kleine petri schalen en kleine stukken cellulose skin: teststukjes cellulose skin zo 'steriel' mogelijk gemaakt zonder ze te autoclaven (heet water, houden boven stoom). Meeste vocht uit de skin laten opdrogen. Daarna de skins goed bevochtigen met LB medium mét glycerine, en inoculeren met Janthinobacterium lividum.
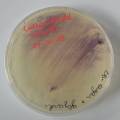
dyeing cellulose skin samples with Janthinobacterium lividum
In the past (2017) I was succesfully growing colonies of Lactobacillus plantarum on cellulose skin drenched with LB medium. The biofilm with these bacteria was then used as a pollution sensor in the project Intelligent Guerilla Beehive, see the researchpage grow your own beehive.
Growing now Janthinobacterium lividum in the incubator, I wanted to research if this violet-blue bacteria will also thrive on microbial-skin-with-food.
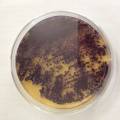
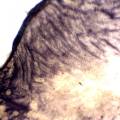
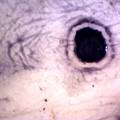
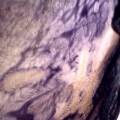
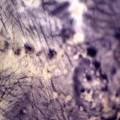
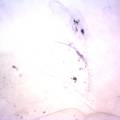
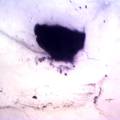
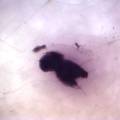
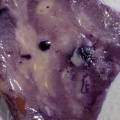
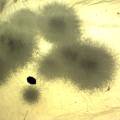
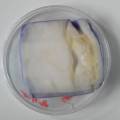
While growing the J.lividum bacteria in the petri's I found already out that they need some drops of glycerol to produce their blue color. So I made some LB growth medium with glycerol and poured it on the samples in the petris dishes, and I inoculated the 2 samples of fresh microbial skin with the J.lividum bacteria. After 2 days I saw yet a blu-ish shine on the wet white skin. I took them out of the incubator and poured some more medium. In one of the samples I injected some medium with a seringue and needle (photo 8). The next day I saw a development inside the skin-sample, but it was not clear if this was the bacteria multiplying or if it was mold. The next days it became clear that it was mold. Probably the needle was not completely sterile when I did the injection. The first sample became more and more blue, and under the microscope it was clear that the colony propagated themselves over the skin. The problem is that they constantly need food (LB) to keep them alive.
dyeing folded cellulose skin with Janthinobacterium lividum
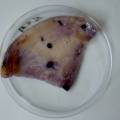
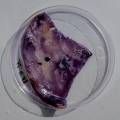
The experiment was to try to color a full-sized cellulose skin with Janthinobacterium lividum. Therefore I put the wet skin in large glass petridishes and I sterilized them in an autoclave bag. When I took the skins out, they were smelling strange - I think that the temp. of 121°C is too high for a microbial skin. I inoculated them with the bacteria, and put enough LB medium as food and put the petri's in the incubator.
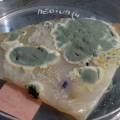
After a few days there was no action - no blue shine, no growth, nothing.
I found out that the Janthinob.lividum do not grow well if there is no glycerol added to the LB (similar for growing the bacteria in a petri dish, glycerol had to be added to the agar nutrient). I made new LB medium with glycerol and I drenched the 2 skins in the medium (in the petri), with the help of a plastic Pasteur-pipette. The pipette was not sterilised, and after a few days mold started to grow on the skins and in the petri. End of the experiment.
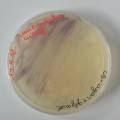
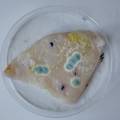
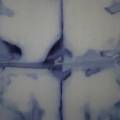
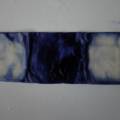
dyeing cotton fabric with Janthinobacterium lividum
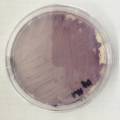
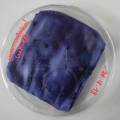
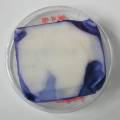
Experiment to color white cotton fabric with Janthinob.lividum bacteria. I sterelized the cotton in the autoclave, in aluminium paper. Than the fabric was drenched with LB medium (with glycerol) and inoculated with bacteria in a sterile setup. After 1à 2 days the bacteria were spreading over the cotton and after 2 days the cotton sample was completely dark blue. The result is a nice blue drawing made by the bacteria on the fabric - the drawing can a little bit be controlled due to the origami folding of the fabric.
workshop bioshades Barcelona - 15/3/2018
dyeing with bacteria: recipe
- ±50×50 cm of silk chiffon or ready to dye cotton fabric
- Janthinobacterium lividum
- glycerine (a couple of drops)
- alcohol
- LB broth (lennox)
- petri dishes
- inoculator loop
- camping gas+holder
- incubator
- gloves
- autoclave bags for sterilisation
- 500 ml glass bottle with lid
- cooking stove
- pressure cooker / autoclave
killing bacteria
- Attached the step by step procedure to inoculate new plates but also growing them on textiles.
- How to make the medium if you don't have it ready made: http://biohackacademy.github.io/bha4/cultivation-media/nutrient-agar/
- To kill the bacteria after use: * wear gloves * take all textiles out of petri dishes and place them in an autoclave bag * take all petri dishes and place them into a bag * put enough water in the pressure cooker, if you use the same pressure cooker i guess ±10 cm * close it, boil 10 min * turn it off leaving pot closed until the steam is gone * throw away autoclave bag with petri dishes * wash all the textiles with washing up liquid
http://wiki.textile-academy.org/bootcamp2017bcn/handson/textilebacteria
https://livingcolour.eu/